Opal
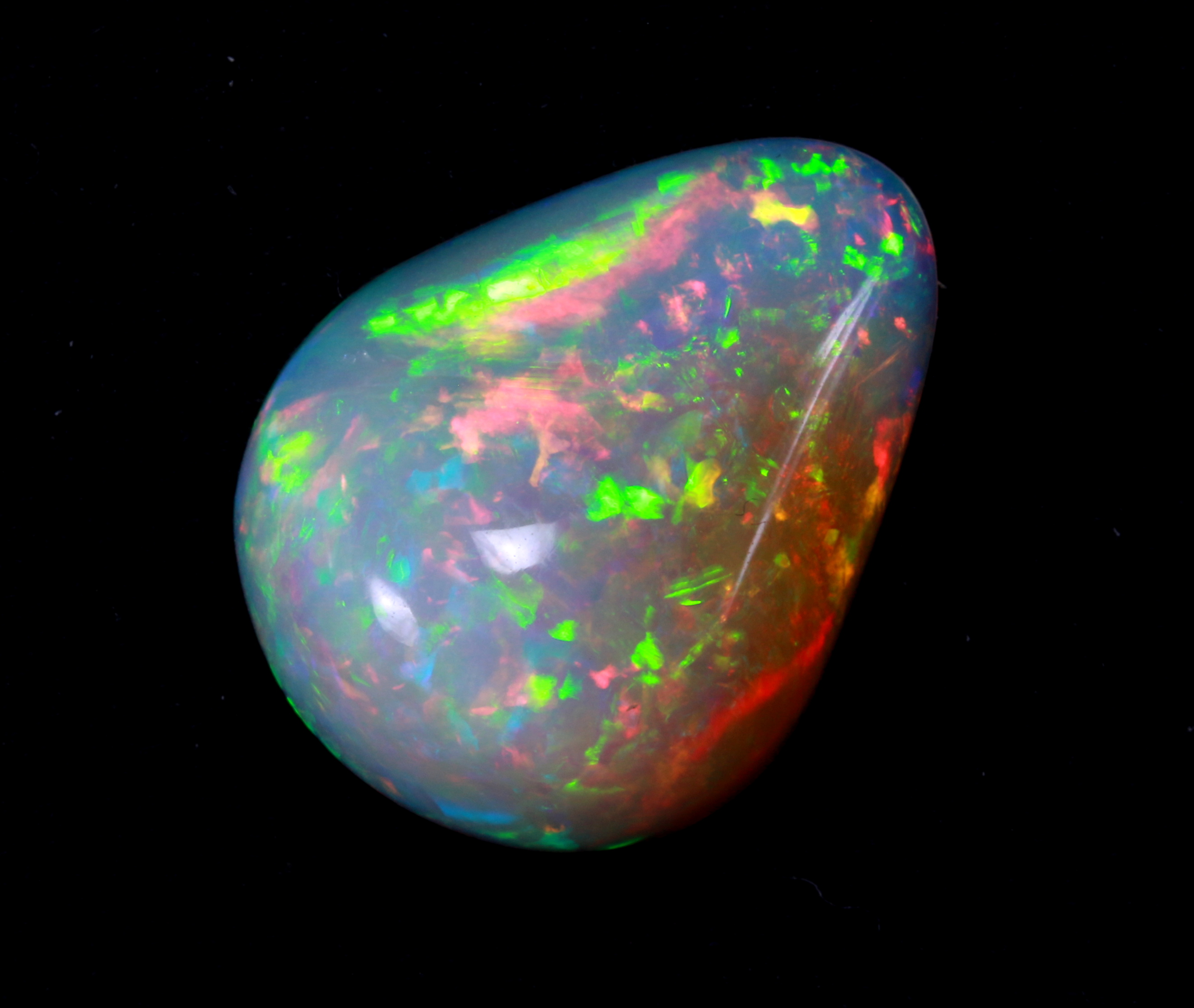
Name: from Pliny (AD) after greek opallios.
Crystallographic characteristics:
amorphous to partially crystalline
Structure: silica gel spheres (150-400 nm diameter), arranged in a cubic densest packing
Chemical characteristics:
Chemical formula: SiO2 x nH2O
Types of opal
|
Precious opal |
opal with colourful play-of-colours, opaque to translucent, with dark (black opal) or lighter (light or white opal) body colour; transparent precious opals are called crystal opal |
|
Matrix opal |
precious opal in host rock |
|
Boulder opal |
matrix opal: Concretion of clay and limonite with thin opal veins |
|
Opal matrix |
opal-bearing rock; its matrix can be dyed to dark colours |
|
Fire opal |
transparent to translucent, red (incorporation of iron hydroxides), sometimes with play-of-colour |
|
Andes opal |
opaque blue to greenish-blue (incorporation of chrysocolla) |
|
Pink opal |
opaque pink (incorporation of palygorskite) |
|
Prase opal |
opaque green (incorporation of nickel minerals) |
|
Girasol |
almost colourless with undulating blue opalescence |
|
Cacholong |
opaque to translucent, porcelaneous to milky white with gray, yellowish or reddish hues |
|
Hydrophane |
porous, often dull opal, absorbs water |
Physical characteristics:
|
hardness |
5½ - 6½ |
|
fracture |
shell-like to brittle |
|
density |
1.74 - 2.22 g/cm3 |
|
refractive index |
n = 1.450 (1.370 - 1.490) |
Optical effects:
|
Play-of-colour |
Diffraction of white light on the three-dimensional arrangement of silica gel spheres. Subsequent interference creates the play-of-colour with pure spectral colours. Individual colour fields are created through areas of different arrangement and size of the silica gel spheres. |
|
Opalescence |
Absorption process by which an opal develops a bluish shimmer when viewed from above, since the blue light is reflected; when viewed through it appears red since the red light is transmitted |
|
Chatoyance (cats-eye effect) |
occasionally (chrysotile fibres) |
Microscopic characteristics: see inclusion gallery
Occurrences: a.o.
|
Australia |
white opal, black opal, boulder opal, Yowah-nut opal |
|
Mexico |
white and black opal, fire opal |
|
Brazil |
white opal, fire opal, opal cats-eyes |
|
Ethiopia |
white opal (often hydrophane!), crystal- or water opal, |
|
Peru |
Andes opal, prase opal, pink opal |
|
other occurrences |
a.o. Indonesia, USA, Slovakia, Mali |
Treatments:
| Kind of enhancement | Identification | Frequency, stability |
|
Dyeing |
sugar charcoal process: dark residues as fine particles |
common |
|
Impregnation |
Furhter methods: |
occasionally stable |
|
Irradiation Alteration of colour |
special fire opals from Brazil change in colour due to irradiation with electrons to blue or green-blue |
rare |
Synthetics:
| Production method | Identification | Specifics |
|
Sedimentation from a mono-disperse silica gel suspension |
exact division of colour fields |
occasionally lower density (1.63 - 1.90 g/cm3) |
Composite opals and imitations:
| Types | Identification | Specifics |
|
Dublets and triplets |
dublets: clear boundary between the crown made of precious opal and the pavilion made of glass / onyx / sandstone / etc triplets: additionally a cover made of a resistant stone |
opal-mosaic-triplets: small pieces of opal on an dark background, embedded in artificial resin |
|
Artificial glasses and plastics |
different physical characteristics (refractive index, density) |
"Slocum" or "Pastoral" |


